Abstract
It is important to prevent pathogenic viral and bacterial transmission within the human environment, especially when there are potential outbreaks caused by the release ancient bacteria that has been trapped in the permafrost. Existing commercial disinfectants have a high environmental footprint. A bioliquid derived from biomass that has been hydrothermally heated liquefied is suggested as a sustainable solution. The bioliquid as prepared showed a high rate of inactivation of pathogenic bacteria and viruses, including H1N1, H5N1, and H7N9 influenza A viruses. Bacillus subtilisVariable. nigerspores, 99.49% Bacillus anthracis. Inactivation Escherichia coli Staphylococcus epidermidisIt has been confirmed that bioliquids can contain low-molecular-weight or low-polarity substances. High temperatures encouraged the production of antibacterial compounds via depolymerization, dehydration reactions and high temperatures. Bioliquid was found to be innocuous by the rabbit skin tests. It also cost $0.04427 per kilogram, which is significantly less than commercial disinfectants. This study highlights the potential for biomass to support biosafety while ensuring greater environmental sustainability.
Biosafety in the environment has received significant attention because of global pandemic or epidemic disease outbreaks that have posed a threat to life security and the global economy. Environment media infected or contaminated with viruses or bacteria are the main vectors of pathogenic microorganisms.1, 2), such as the transmission of influenza A virus via the fecaloral route (3). As permafrost thaws as a result of global warming, ancient bacteria and viruses such as Bacillus anthracisThe environment can be contaminated and cause severe damage to the health of people.4, 5). These events could become more common as global warming continues to accelerate.
It is essential to prevent the spread of diseases by disinfection. The current commercial disinfectants have a number of drawbacks. These include toxic byproducts as well as a high carbon footprint from complex preparation processes.6). Permafrost is found in ecologically fragile regions. A large use of commercial disinfectants like sodium hypochlorite could potentially lead to carcinogenic trihalomethanes.6). Commercial disinfectants also rely heavily upon nonrenewable materials, which is not conducive for sustainable development. Some commercial disinfectants do not work well against spores (such a B. anthracis) of bacteria (7), due to its single component which also might spread a superbug (8). It is therefore urgent that renewable, effective, and environmentally-friendly disinfectants be developed to ensure biosafety in human environments.
Biomass is readily available and can be regenerated. It has typical multiantibacterial structures such as phenols or ketones.9). Although it is a bioliquid derived from biomass pyrolysis, also known as pyroligneous acids, it does contain antibacterial components.10Its effective concentration is low (11). This problem can be overcome using the combination of wet processing and subcritical hydrothermal, which is a highly efficient technology to crack biomass at low temperatures.12). This study examined the effectiveness of a renewable disinfectant made from biomass. This study shows that biomass can be used to support biosafety in human environments.
Results and Discussion
Compounds with low Molecular-Weight and low-Polarity have excellent antibacterial activity.
Escherichia coli(gram-negative) Staphylococcus epidermidisGram-positive bacteria are the most common pathogenic bacteria.13). The inactivation rate is E. coli S. epidermidisBioliquid can reach up to 100%Fig. 1 A B) due to damage to the cell wall (Fig. 1C). The half-maximal effect concentration (EC).50) was 2,015, 1,426, and 1,452 mg/L for E. coli782 mg/L, 196 mg/L, and 137mg/L S. epidermidisFor the bioliquid produced at 210C to 270C and 335C respectively. These results indicate that the bioliquid at high temperatures had better antibacterial activity.
” data-icon-position=”” data-hide-link-title=”0″>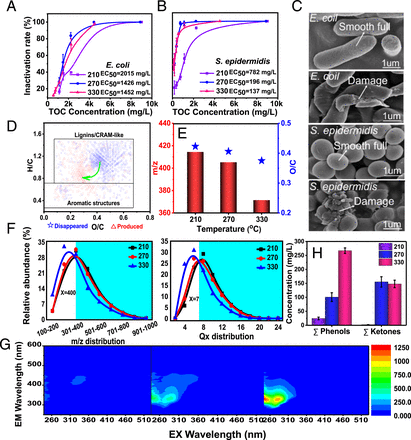
Bioliquids are tested for antibacterial components E. coli S. epidermidis. (A B) Inactivation rate of E. coli S. epidermidisBioliquid is used to treat the problem. (C) SEM of E. coli (Top) and S. epidermidis (Bottom) before (first image) and after (second image) treatment by the bioliquid produced at 270C. (D) Van Krevelen diagram of CHO for the bioliquid produced at 330C compared to 210C. (E) The m/zO/C ratio, and (F) relative abundance of m/zDistribution of Ox. (G) Fluorescence EEM spectra and (HThe bioliquid’s phenol and ketones concentrations are shown in the green arrow. The green arrow DThis indicates the direction of the H/C and O/C moleratie changes for the bioliquid produced at 325C as opposed to 210C. TOC, total organic matter.
The molecular structures of bioliquid were qualitatively examined in order to identify the antibacterial compounds. The Van Krevelen diagrams of CHO chemicals suggest that the hydrothermal temperature promoted the transfer of compounds from lignin/carboxylic-rich alicyclic moleculelike structures to aromatic structures, as indicated by the downward-moving H/C values (Fig. 1D). Furthermore, low-molecular weight and low-polarity chemicals were easy to produce at high temperatures. This was shown by the decrease in m/zO/C ratios range from 414.5-371.5 and from 0.423- 0.375, respectivelyFig. 1E). The relatively high levels of both compounds are a result. m/z> 400 and oxygen number 7 were clearly transformed into lower-molecular and lower-oxygen substancesFig. 1F). This happened because lignocellulose was slowly depolymerized. It also dehydrated, leading eventually to bond breaks and oxygen separation at an increasing hydrothermal heat.12). The excitationemission matrix (EEM) results further suggested that substances at 239/330 nm and 275/312 nm (excitation/emission) were related to phenol-like compounds produced by lignin depolymerization (12, 14). Fluorescence intensity rose with increasing hydrothermal temperature. This indicated that fluorescence concentration was increasing (Fig. 1G). Gas chromatography mass spectrometry, (GC-MS), is also available.Fig. 1H) illustrated that the concentrations of low-boiling phenols and ketones (low-molecular-weight compounds) and those of the typical antibacterial components (15) were high for bioliquid yielded at 270C and 330C. It can be concluded that bioliquid produced at high temperatures (270C and 330C), had better inactivation results due to the high concentrations of low-molecular weight and low-polarity chemicals.
The Bioliquid’s broad-spectrum potential to inactivate a variety of pandemic causing pathogens is impressive.
To investigate the pandemic response and broad-spectrum potential, the inactivation and destruction of the influenza A virus (H1N1,H5N1,and H7N9) was done. B. anthracisA biosafety laboratory level 3 tested bioliquids produced at 270C. Quartz sand (the main ingredient of soil) was simulated as H1N1 masks. B. anthracis carriers. Cell viability was 100% after bioliquid was reduced to less than 1.23% for MadinDarby Canine Kidney cells (MDCK).Fig. 2A). The inactivation rates of H1N1 and H5N1 (masks and quartz sand carriers), H7N9, and H5N1 were all 99.99%Fig. 2B), and that of B. anthracis99.59% (quartz sand was used as carrier). These results strongly suggest that H1N1 was not inactivated by carriers. Bioliquid can prevent the transmission of pandemic-causing bacterias and viruses. Additionally, the inactivation of H1N1 was not affected by carriers. Bacillus subtilis var. nigerThe 99.99% spores, which is a common challenge bacterium that is used to test the disinfection effectiveness of bioliquid, indicate that it has a broad range of disinfection properties. The bioliquid was also injurious to rabbit skinFig. 2 C DThis suggests that bioliquid is safe for animals and people who accidentally touch it. Bioliquid was also found to be safer than hypochlorite or phenolic disinfectants in terms of earthworm toxicity.Fig. 2E). The inactivation rates E. coli S. epidermidisBioliquid derived primarily from different types biomass had a 99.9% success rate, which indicates that bioliquid derived largely from all types biomass has a high antimicrobial capacity. The result of EC50Further, it was suggested that biomass with high levels of lignocellulose or low ash may be preferred for inactivation effects.Fig. 2 F G). This bioliquid’s potential production quantities and costs were estimated at 113.02 Million Tonnes and $0.04427/kg. Large-scale production is possible because of the availability and low cost renewable materials. These materials are more cost-effective than commercial disinfectants.Fig. 2H).
” data-icon-position=”” data-hide-link-title=”0″>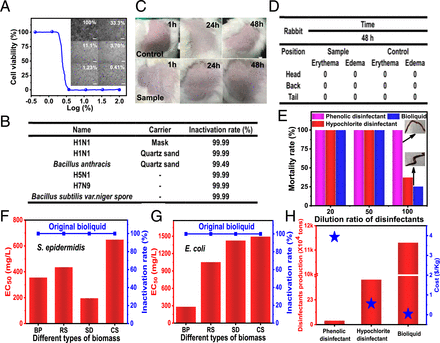
Disposable skin tests and inactivation of epidemic-causing pathogens using bioliquid at 270C (A) Cytotoxicity of the bioliquid to MDCK cells. (Scale bars, 20 m.) (B) Inactivation of H1N1, H5N1, H7N9, B. anthracis, Bacillus subtilisVariable. niger spores. (C DDisposable skin tests for rabbits (E) The earthworm toxicity evaluation. (F G) Bioliquid derived from different types of biomass for S. epidermidis E. coli inactivation. (H) The potential production of the bioliquid as a disinfectant, and the corresponding cost per kilogram in China.
The bioliquid developed is an excellent candidate to prevent an outbreak and establish better biosafety in human environments by inactivating H1N1,H5N1, and H7N9. B. anthracis. This bioliquid is also versatile and low-cost, since it is made from renewable biomass. This is a benefit for large-scale projects. The potential of this study should be expanded upon to further investigate the separation method for enrichment of low molecular weight and low-polarity compounds. This will allow bioliquid to be commercially available.
Materials and Methods
Bioliquid was obtained from biomass by hydrothermal liquid liquefaction at different temperatures. Its effectiveness against highly pathogenic microorganisms like H1N1, H5N1, and H7N9 is also demonstrated. B. anthracisThe results were analyzed. The correlation between inactivation effects of bioliquid and its molecular structure was also determined by inactivating E. coli S. epidermidis. For more information, please visit SI AppendixFor the extended materials or methods.
Data Availability
All data from the study are included in the article. SI Appendix.
Acknowledgments
This research was supported in part by the National Natural Science Foundation of China (Grant 21876030) and Natural Science Foundation of Shanghai (19ZR1403800).
Footnotes
- Accepted November 11, 2021
-
F.Y. S.Z. and X.Z. F.Y.., S.Z.. and X.Z. Contributed new reagents/analytical tools; F.Y. Zhao, T.Q. Wang Zhao, Y.C. X.M. L.L.H.S.Y.Y.Z.S.Z. and X.Z. F.Y.Y.Z.S.Z., X.Z. The paper was written by F.Y.
-
The authors declare that they have no competing interests.
-
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2106843119/-/DCSupplemental.
- Copyright 2022 to the Author(s). Publication by PNAS.
